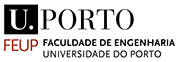Meeting – DiscoveryMat and Pleco, two open source and low-cost scientific instruments for Cultural Heritage applications
27 - 28 May 2021 - Toulouse, France

Staff of Materia Viva who attended the meeting / workshop: on the left, Caroline Moreau and on the right, Monique Drieux, director of Materia Viva, in the middle, Caroline Moreau and on the right Typhaine Brocard-Rosa
Location: Materia Viva (Conservation laboratory), 27 Rue Bernard Délicieux, 31200 Toulouse, France
Start and end date: 27/05/2021 to 28/05/2021
Grantee name: Dr. Christian Degrigny, Haute Ecole Arc Conservation-restauration, 2000 Neuchâtel, Suisse
Purpose of the meeting / workshop
Materia Viva is a conservation laboratory in the South West of France. It specialises in the treatment of archaeological and historical artefacts, including metals. In recent years, Materia Viva has started to clean silver artefacts which are often tarnished.
HE-Arc CR has developed the open-access DiscoveryMat application and open source Pleco electrolytic pencil to analyse historic metal artefacts and their corrosion products.
The purpose of the meeting / workshop was to demonstrate the possibilities of the analysis tools in a conservation laboratory and to see if these portable and easy-to-use systems meet the expressed needs.
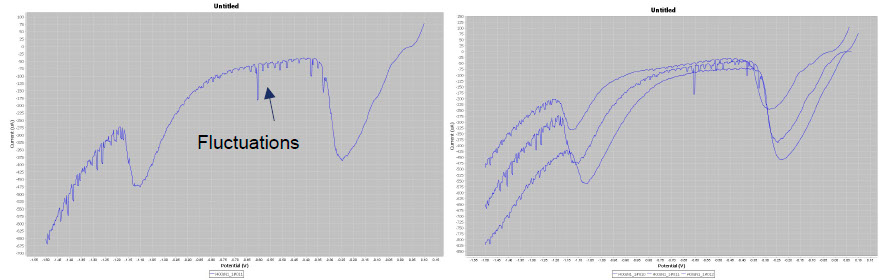
C. Degrigny brought his instrumentation from the Haute Ecole Arc Conservation-restoration, Neuchâtel while the local Metrohm agency provided a DropSens potentiostat. The artefacts were provided by Materia Viva.
Description of the work carried out during the meeting / workshop

The device used to analyse silver tarnish on the lower part of the base of a silver-based monstrance.
C. Degrigny first demonstrated the capabilities of the Pleco. The electrolytic pencil contains a 3-electrode electrolytic cell that allows Linear Sweep Voltammetry (LSV) plots used to identify the presence of corrosion products (see below). Furthermore, the system is equipped with a pumping system that constantly supplies (15mL/min) and extracts (50mL/min) the electrolyte.

Left, a cross-section of the Pleco electrolytic cell, and right, the analysis in progress.
During the workshop, LSV plots were used to identify the silver tarnish that develops on silver-sbased objects. As shown below, the typical constituents of silver tarnish appear: AgCl and Ag2S. AgCl is revealed first after Ecorr (corrosion potential) at around 0V/glossy carbon (around 400mV/SHE) while Ag2S appears around -0.75V/GC. Their characteristic reduction potentials correspond to the beginning of the peaks. The potential of the peak maximum depends on the thickness of the compound to be reduced.
LSV plot in the cathodic domain showing from Ecorr two reduction peaks characteristic of the silver tarnishing.
Secondly, the DiscoveryMat application for the identification of metal artefacts was examined. The analysis is qualitative and is currently only possible on copper and aluminium-based alloys. The principle is based on the monitoring of Ecorr versus time in three different solutions (Evian water, KNO3 and sodium sesquicarbonate both at 1% w/v) using a voltmeter controlled by DiscoveryMat application. Sodium sesquicarbonate is an equimolar buffered solution (pH=10) of NaHCO3 and Na2CO3. A reference electrode (RE) in a junction tube (JT) containing the same solution as the one to test is located above the metal surface. A drop of the test solution is inserted between the metal surface and the RE / JT, allowing the Ecorr to be monitored (see pictures below). The monitoring is carried out first for 5 min (2 plots) to check the reproducibility of the measurements. The Ecorr vs time plot to consider is continued for 15 min. The three 15 min plots in each solution are then compared to similar plots in the DiscoveryMat datatase.

Left, inserting the test solution at the interface between the metal surface and the RE / JT system and right, data collection in progress.
Description of the main results obtained
- LSV plots and their application to the identification of silver tarnish -
- Base of a monstrance
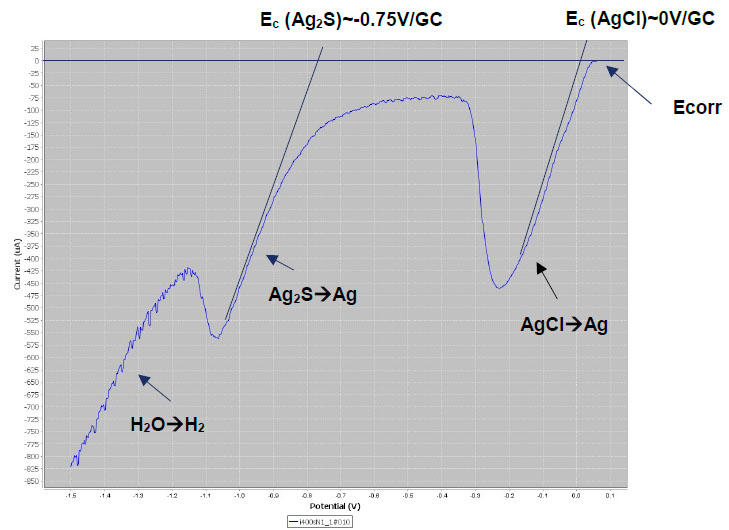
LSV plots are suitable for identifying silver tarnish. Below left, we see the typical reduction peaks of AgCl and Ag2S on the lower parts of the monstrance base. Fluctuations in the current are observed. These are due to the operation of the Pleco (electrolytic flow that changes the exposed metal surface). Below right, it can be seen that the LSV plots are quite reproducible.
LSV plots on the lower parts of the monstrance base. Left: fluctuations due to the electrolyte flow. Right: overlaying of LSV plots to test the reproducibility of the measurements.
Additional LSV plots were made on more decorated surfaces of the monstrance base. This time the AgCl peak appears small compared to the Ag2S peak.
LSV plots on the decorative surfaces of the monstrance base.
The Pleco is not only an analysis tool but also a cleaning system. To clean silver tarnish, the user applies a potential to the object corresponding to the maximum of the cathodic peak of Ag2S → Ag. In doing so, AgCl, which has a lower reduction potential, is automatically reduced. Conservators are not only interested in defining the maximum of the Ag2S reduction peak but also in the duration of the reduction process. Chronoamperometric plots are used for their determination. In the case below, 2 min are needed to obtain a complete reduction. Once the cathodic potential of the electrolytic cleaning and its duration have been determined, the conservator can clean the silver tarnish safely. To do this, Material Viva staff change the Metrohm potentiostat to a more robust EGG potential that operates in the 0-1 A range and the Pleco is used dynamically (see picture below).
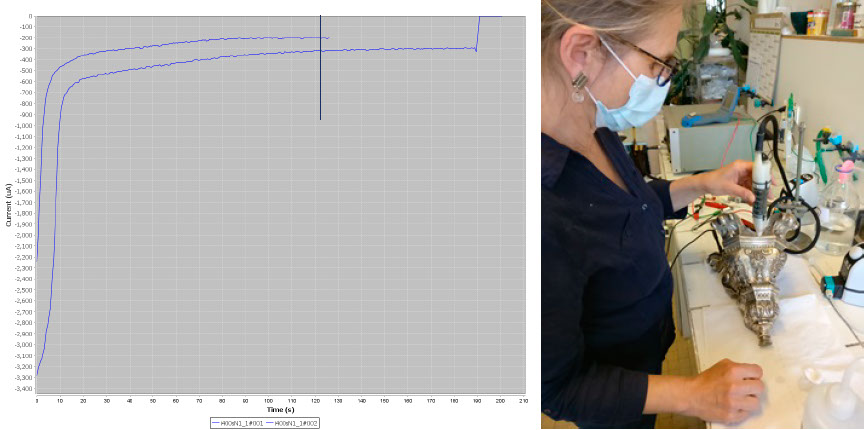
Left, chronoamperometric plots obtained at -1.45V/GC and right, the Pleco used in dynamic mode.
|Incense burner
Again, the LSV plot on the top element of the incense burner shows the typical constituents of silver tarnish.
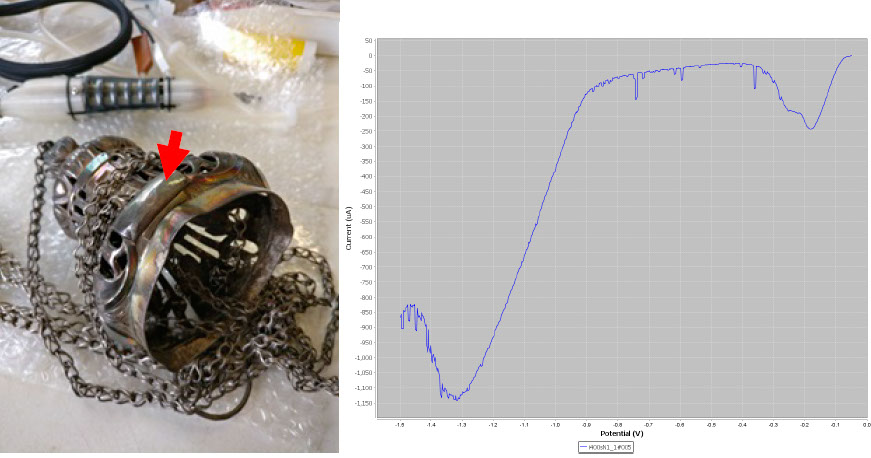
LSV plot on a section of the top element of the incense burner.
- Candlestick
This object was selected since it has elements made of silvered copper alloys (button).

Left, body of the candlestick put upside down and left, LSV on its top button (right).
As expected the LSV plot on the body (below left) gives the typical AgCl and Ag2S peaks. The silvered copper-based button gives two additional reduction peaks (waves, below right) attributed to Cu2O → Cu and Cu2S → Cu.
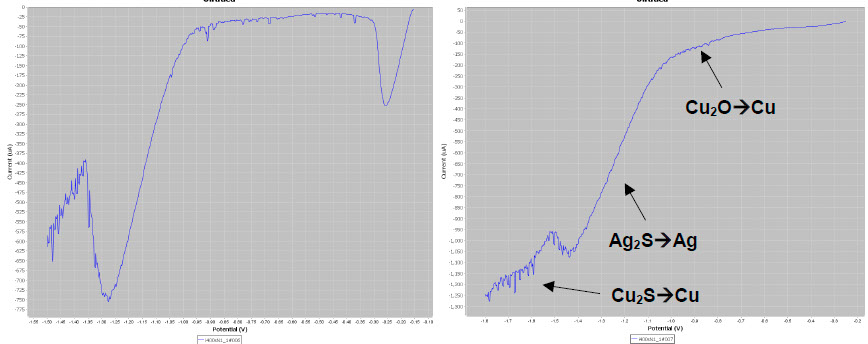
LSV plots on different elements of the candle holder.
The identification of the presence of Cu2O and Cu2S is essential for conservators as their reduction causes black spots that must be avoided. To prevent this side affect, conservators perform a preliminary immersion of the artefacts in a copper compound chelating agent (EDTA tetrasodic salt).
- Analysis of a metal artefact with DiscoveryMat -
DiscoveryMat application was tested on a candlestick made from a copper alloy to identify it.
DiscoveryMat application used to analyse the metal of a candlestick.
The three Ecorr vs time (15min.) plots obtained first in Evian water and then in KNO3 and sodium sesquicarbonate, both at 1% w/v are given below.
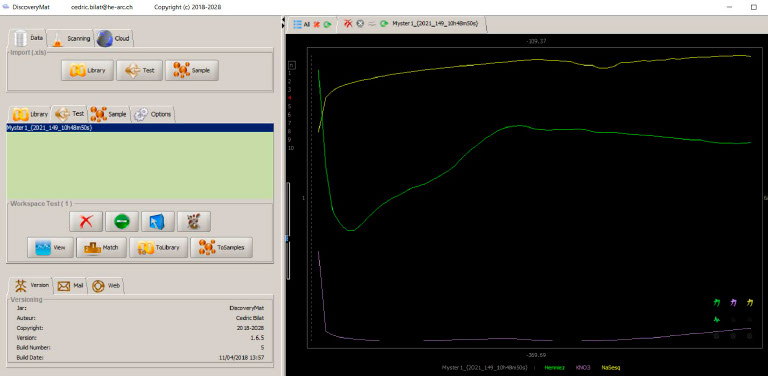
Compilation of Ecorr vs time plots in Evian water, KNO3 and sodium sesquicarbonate 1%w/v on the DiscoveryMat application.
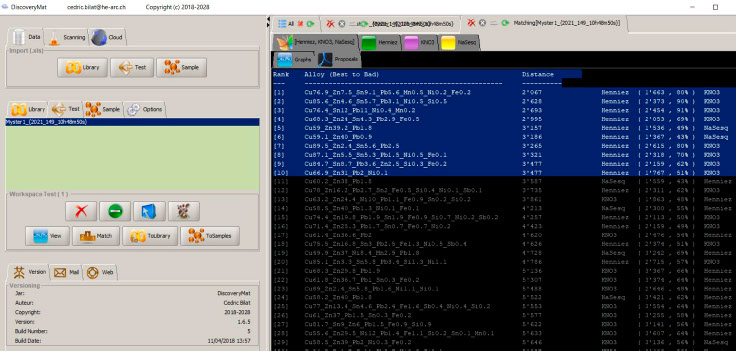
Matching of the plots with those of the DiscoveryMat database, proposal mode.
Among the first 10 composition proposals given by the DiscoveryMat application, there are 4 quaternary alloys (Cu/Sn/Zn/Pb) and 4 leaded brasses.
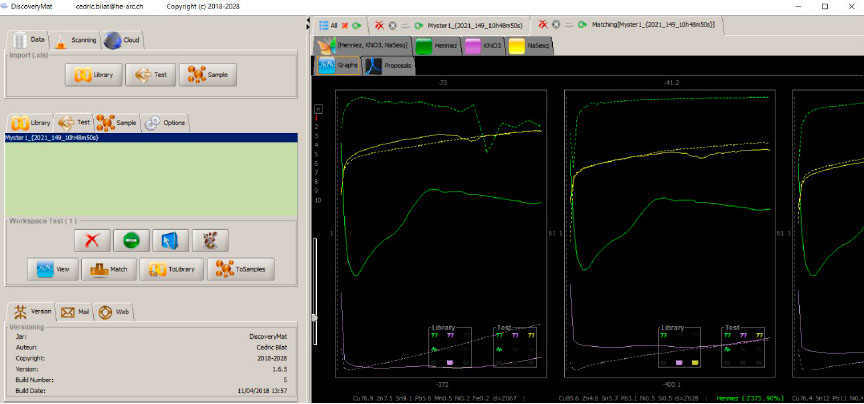
Matching of the plots with those of the DiscoveryMat database, graph mode.
Comparison of the graphs clearly shows that there is no material in the database close to our alloy but that the closest plots are those of quaternary alloys (CuZn 7.5 Sn 9.1 Pb 5.6 or CuZn 4.6 Sn 5.7 Pb 3.1). The exact composition of the metal can only be given by XRF elemental analysis.
Conclusions and future collaborations
The meeting / workshop organized at Materia Viva conservation laboratory was a success as we clearly demonstrated the possibilities of the two portable, easy-to-use and low-cost tools used.
DiscoveryMat gave an idea of the composition of the tested artefact. We were hesitating between a bronze or brass alloys. The analyser indicated that the metal is most likely a quaternary alloy.
The Pleco electrolytic pencil confirmed that the silver tarnish that developed on the tested objects is mainly constituted of Ag2S, while AgCl and in some cases Cu2O or Cu2S can be found. Materia Viva staff were not aware of the large amount of AgCl on their artefacts. Furthermore, the identification of copper corrosion products is essential to define an appropriate electrolytic cleaning preventing the formation of black spots.
C. Degrigny and M. Perraudin, representative of the Metrohm agency in Toulouse, left their instrumentation (Pleco and potentiostat) with Materia Viva staff for a week so that they could continue to practice.

.png?crc=4271212285)